Rare Disease & Gene Therapy: Specialty Pharmacy Distribution and Manufacturer Engagement
Highlights of the report:
Download a PDF of these Highlights
Specialty pharmacies are developing robust and unique capabilities to support the growing market of rare disease & gene therapies, and can serve as strong partners in patient access and adherence. HIRC’s report, Rare Disease & Gene Therapy: Specialty Pharmacy Provider Distribution & Manufacturer Engagement, examines issues in dispensing and distribution networks, and evaluates best-in-class manufacturer support. The report addresses the following:
- What do specialty pharmacy providers (SPPs) report as their access to rare disease & gene therapy medications? How does this vary by SPP ownership type (e.g., PBM/health plan-owned, independent, health system)?
- What are the trends in manufacturer specialty pharmacy distribution networks for rare disease & gene therapies?
- What are SPPs' capabilities in rare disease dispensing? How does this differ by ownership type?
- What contracting approaches are manufacturers engaging in to support SPPs' clinical and data collection activities for rare diseases?
- Which manufacturers are considered best-in-class in SPP rare disease engagement?
- What opportunities exist for manufacturers to better support SPPs in rare disease?
Key Finding: Manufacturers are intentional in their network selection, collaborative initiatives, and contracting engagement with specialty pharmacy partners to best meet the needs a given rare disease product and patient population.
Specialty Pharmacy Rare Disease Dispensing Overview. Specialty pharmacy providers report access to 19 rare disease or gene therapy products on average in 2024, with PBM/Health plan-owned SPPs reporting access to a larger number of products compared to independent/other and health system-owned SPPs, though all report an increase in number of products since 2022. Roughly 57% of the rare disease medications dispensed by SPPs are self-administered, while professionally-administered medications account for 43%.
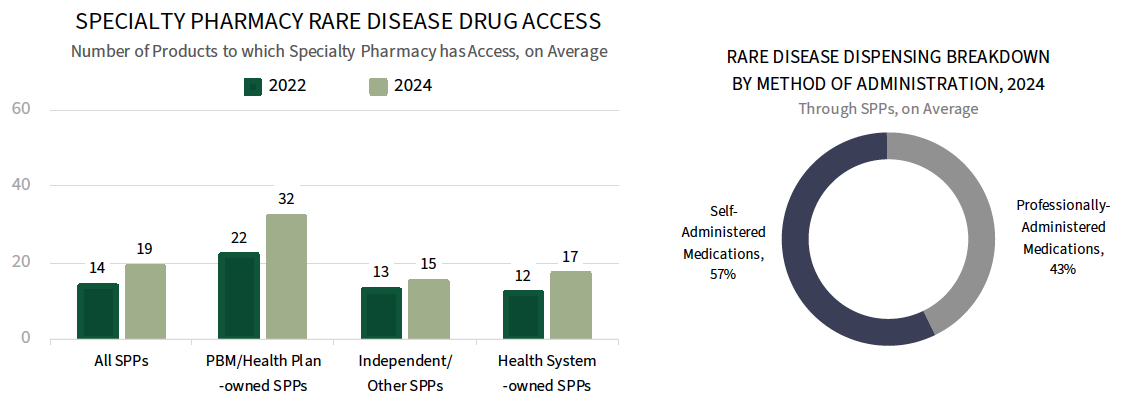
The full report examines SPP rare disease dispensing trends across 20 therapeutic areas, as well as considerations for network selection.
Specialty Pharmacy Contracting Approaches in Rare Disease. Contracts between manufacturers and specialty pharmacies are relatively common to support rare disease and gene therapy dispensing. HIRC's research examines the prevalence of four unique contract types: 1) Enhanced Clinical Services contracts, 2) Enhanced Data Services contracts, 3) Upfront/Off-Invoice Discounts, and 4) Performance-based Rebates. Enhanced clinical service contracts are most common overall and reported most often by dispensing SPPs for rare kidney disease, Fabry disease, and gene therapy.
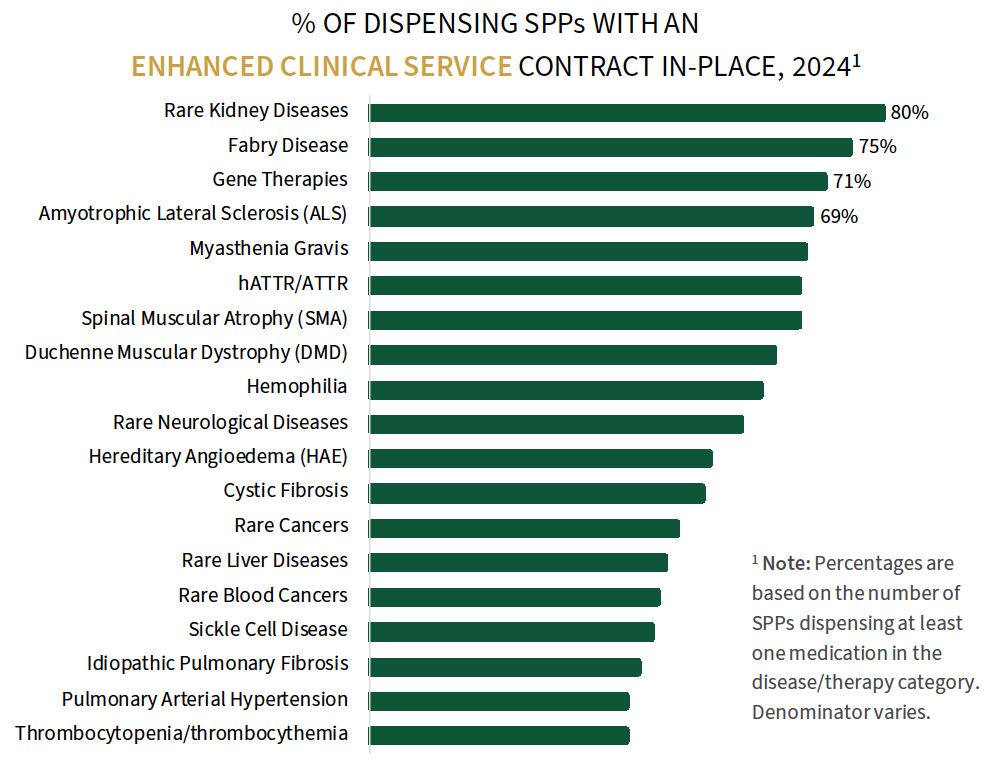
The complete report examines the contracting environment in detail across 20 rare disease areas and by SPP ownership type.
Novartis, Genentech, & Pfizer Among Leaders in Rare Disease Engagement with SPPs. Plans were asked to consider and provide a best-in-class manufacturer nominations across four rare disease engagement parameters as noted below. Novartis, Genentech, and Pfizer are consistently among those nominated as best across categories in 2024.
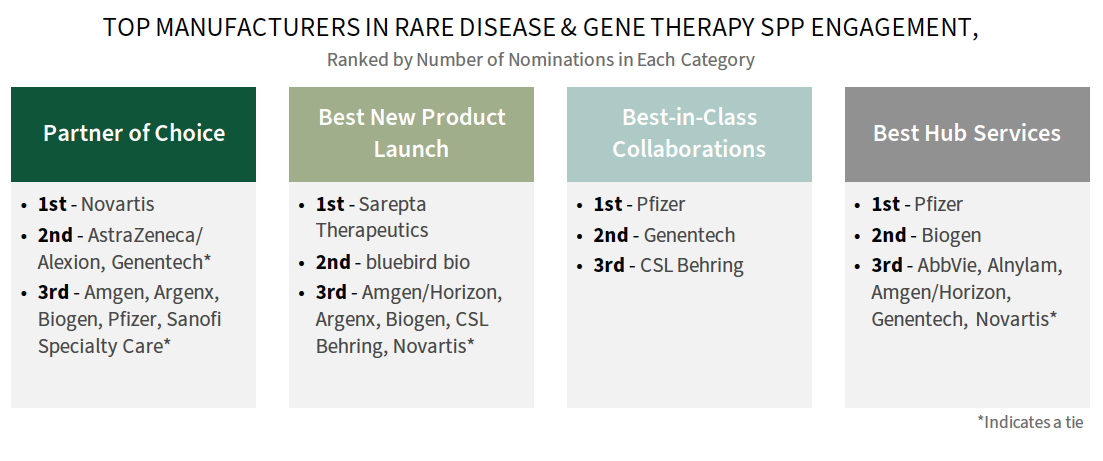
Note – Because rare disease distribution networks are often quite small, there is likely a high amount of variability around which manufacturers a specific specialty pharmacy is interacting with.
Research Methodology and Report Availability. In July/August, HIRC surveyed 40 PBM/health plan-owned, health system-owned, and independent/other specialty pharmacy providers. Online surveys and follow-up telephone interviews were used to gather information. The Rare Disease & Gene Therapy: Specialty Pharmacy Provider Distribution & Manufacturer Engagement report is part of HIRC's Special Reports Series, and is now available to subscribers at www.hirc.com.
Download a PDF of these Highlights
Download Full Report (Subscribers only) >


