Rare Disease & Gene Therapy: Commercial MCO Management, Financing, & Mfr. Engagement
Highlights of the report:
Download a PDF of these Highlights
A number of innovative rare disease and gene therapy treatments have entered the market, requiring novel utilization management, financing, and contracting approaches. HIRC’s report, Rare Disease & Gene Therapy: Commercial Health Plan Management, Financing, and Manufacturer Engagement, examines plans' handling of high and ultra high-cost therapies across 20 rare disease states and evaluates best-in-class manufacturer support. The report addresses the following questions:
- How are plans working to manage the cost and utilization of rare disease and gene therapies in 2024/2025?
- What specific utilization and formulary management tactics are applied across 20 unique rare disease categories?
- Which financing mechanisms and protection programs are plans using/exploring for rare disease medications?
- What is the nature of the contracting environment across 20 broad rare disease states? How interested are commercial MCOs in novel contracting arrangements for rare disease & gene therapies?
- How are manufacturers supporting commercial plans and patients in rare disease?
Key Finding: Prior authorization is among the primary tools commercial plans use to manage access to and appropriate use of rare disease medications; in some classes there are enough products for plans to designate preferred products and begin to leverage step therapy.
Prior Authorization Criteria and Value-based Contracts Top Commercial MCOs' Efforts to Manage Rare Disease Spend. Commercial MCOs are managing the cost of rare disease and gene therapies mostly through a combination of traditional utilization management tactics and novel financing/contracting mechanisms. Specifically, plans are implementing strong PA clinical requirements, often considering the clinical trial inclusion/exclusion criteria and focusing on reauthorizations to ensure response to therapy. Plans are also seeking value/outcomes-based contracts to accompany ultra-high cost therapies through the form of warranties, rebates, or reimbursement adjustments.
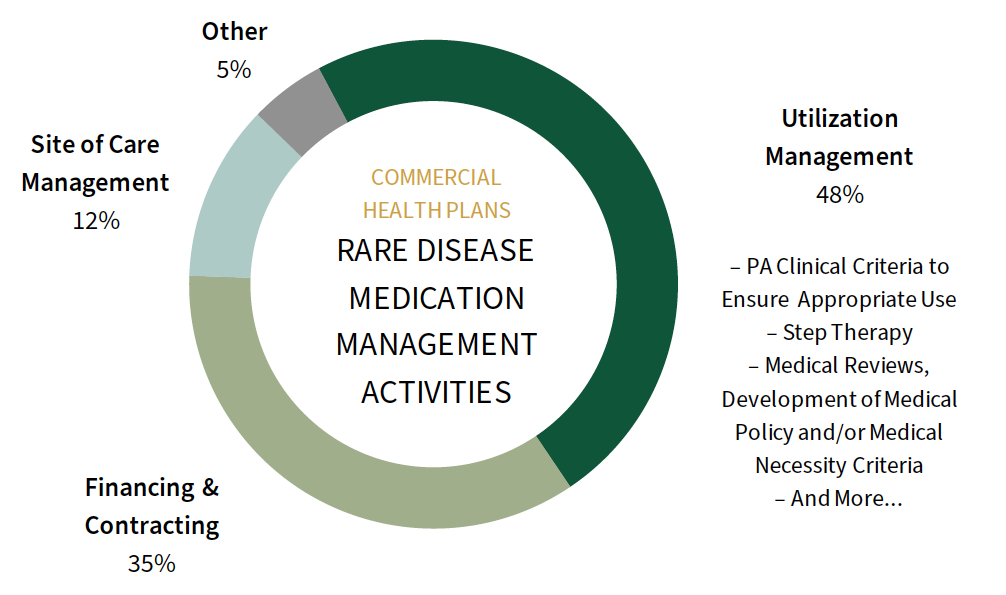
The full report examines management activities in detail across 20 rare disease categories.
Financing & Contracting Considerations in Rare Disease. Commercial MCOs are evaluating a variety unique financial protection programs, benefit designs, and other methods to finance rare disease & gene therapies, discussed in the complete report. When it comes to contracting with manufacturers, the approaches vary by therapy type. Traditional access-based contracts are used, and a higher prevalence of risk/outcomes-based contracts are observed for gene therapy compared to traditional specialty drugs. Contracts are most often reported in hemophilia and pulmonary arterial hypertension (PAH).
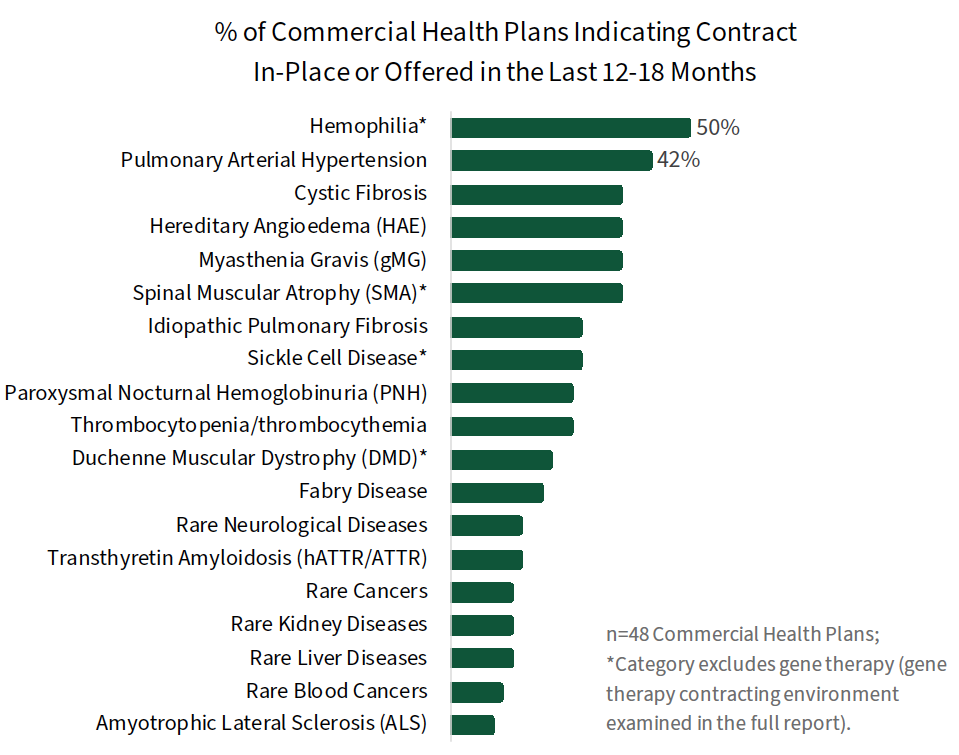
The full report examines the rare disease contracting environment in detail, as well as plans' interest in prevalence-based rebates, value/outcomes-based contracts, and pay-over-time arrangements.
Genentech and Alnylam are Among Leaders in Rare Disease Engagement with Commercial MCOs. Plans were asked to consider and provide a best-in-class manufacturer nomination across three rare disease engagement parameters as noted below. Genentech, Alnylam, Vertex, Pfizer, Argenx, and Novartis are among those nominated as best across categories. Many of the leading manufacturers in rare disease engagement in 2024 had recent product launches, where products are accompanied by significant support services, novel contracting arrangements, and site of care assistance.
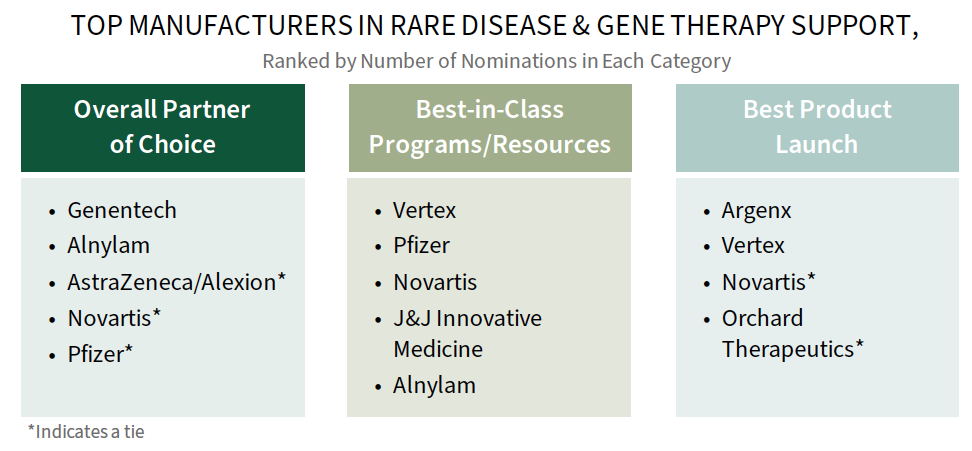
The complete report provides the full listing of manufacturers recognized, drivers of best-in-class nominations, and a commercial MCO needs assessment.
Research Methodology and Report Availability. In September, HIRC surveyed 48 pharmacy and medical directors from national, regional, and BCBS plans representing 110 million lives. Online surveys and follow-up telephone interviews were used to gather information. The Rare Disease & Gene Therapy: Commercial Health Plan Management, Financing, and Manufacturer Engagement report is part of HIRC's Special Report Series, and is now available to subscribers at www.hirc.com.
Keywords: Commercial Health Plans, Rare Disease, Gene Therapy, Financial Protection Programs, Value-based Contracting, Risk/Outcomes-based Contracting, Site of Care, Center of Excellence, ICER, Copay Maximizer, Copay Accumulator, Alternative Funding, Partner of Choice, Launch, Program/Resource Offering, Market Access Dashboard, Cystic Fibrosis, Duchenne Muscular Dystrophy (DMD), Fabry Disease, Hereditary Angioedema (HAE), Spinal Muscular Atrophy (SMA)*, Transthyretin Amyloidosis (hATTR/ATTR), Hemophilia, Paroxysmal Nocturnal Hemoglobinuria (PNH), Sickle Cell Disease, Thrombocytopenia/thrombocythemia, Rare Blood Cancers, Rare Cancers, Amyotrophic Lateral Sclerosis (ALS), Idiopathic Pulmonary Fibrosis, Myasthenia Gravis (gMG), Pulmonary Arterial Hypertension, Rare Kidney Diseases, Rare Liver Diseases, Rare Neurological Diseases
Download a PDF of these Highlights
Download Full Report (Subscribers only) >


